We decided to mine the biomarkers being used in clinical trials for interesting test development opportunities, and we began with Oncology as the first therapeutic area to explore.
Our basic methodology was to identify biomarkers that are in regular (and hopefully growing) use in Oncology, and that are also being used in at least one other therapeutic area as well. The goal with the latter criterion was to try to de-risk potential opportunities by having more than one possible use for any new test.
Our analysis began with a list pulled from BiomarkerBase of every biomarker identified as being used in clinical trials initiated within the last 2.5 years.
We filtered that list to include only those biomarkers for which no FDA-approved test is yet available. We also filtered for the number of Oncology trials in which each biomarker is being used (at least four), and for the total number of therapeutic areas in which each biomarker is being used (at least one outside of Oncology). We also ensured that each biomarker is being used in more than three trials in all therapeutic areas.
We then set a range for the total number of clinical studies that have been published for each biomarker, under the hypothesis that biomarkers with less publications, but which are well-represented in recent clinical trials, might be particularly hot.
With these set of filters in place we generated a list of 10 biomarkers that could represent interesting opportunities for new tests, or for more aggressive promotion of existing tests.
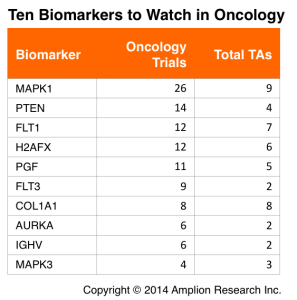
This table lists 10 biomarkers that could represent good opportunities for test developers. The Oncology Trials are the total number of trials initiated within the last 2.5 years that disclose one of the biomarkers in the CT.gov record. Total TAs is the total number of therapeutic areas (Oncology included) in which each biomarker is being used.
Reviewing additional details about each biomarker provides the ability to further prioritize this list. Here are some brief summaries describing each biomarker.
MAPK1
This well-characterized kinase is most associated with cancer, but is now being used in clinical trials in a variety of therapeutic areas, with Neurology being the most prominent.
- Existing test providers: none among Labcorp, Quest, and ARUP
- Available RUO assays: IHC, PCR, ELISA
PTEN
This tyrosine phosphatase was first published in a clinical study in 1999, and has appreciated 27% annual growth in publications that include it over the last several years.
- Existing test providers: Quest, ARUP
- Available RUO assays: IHC, ELISA
FLT1
This tyrosine kinase was first published in a clinical study in 2000, and in recent years there has been a 35% annual increase in the number of clinical studies that include FLT1, so this is clearly a biomarker on the rise.
- Existing test providers: none among Labcorp, Quest, and ARUP
- Available RUO assays: IHC, PCR, ELISA
H2AFX
It has only been 7 years since this histone was first described in a clinical study, making it the second most recent in this list to make its way into clinical investigation.
- Existing test providers: none among Labcorp, Quest, and ARUP
- Available RUO assays: IHC, ChIP, ELISA
PGF
Placenta growth factor has been used clinically since 1973, and is easily the oldest of the biomarkers in this list. It also seems to be losing favor a bit as a cancer biomarker given recent publication history, so I almost didn’t include it. In the end it made the cut because of the possibility that it is gaining favor in other therapeutic areas (especially pre-eclampsia – see Alere’s dedicated site for this biomarker).
- Existing test providers: none among Labcorp, Quest, and ARUP
- Available RUO assays: ELISA
FLT3
Another tyrosine kinase, FLT3 was cited in 23 clinical studies in 2013, and numerous drugs under development are FLT3 inhibitors.
- Existing test providers: Labcorp, Quest, and ARUP all offer tests
- Available RUO assays: Activity, PCR, ELISA
COL1A1
This gene encodes for connective collagen, and is being used in a diverse variety of therapeutic areas.
- Existing test providers: Labcorp, Quest, and ARUP all offer tests
- Available RUO assays: ELISA
IGHV
IGHV is an immunoglobulin region associated with leukemia that has only been cited in clinical studies since 2005, but which was included in 9 such studies in 2013 alone.
- Existing test providers: ARUP
- Available RUO assays: PCR, ELISA
AURKA
The Aurora a kinase is the most recent on the list in terms of citation in a clinical study, having first appeared in 2008.
- Existing test providers: none among Labcorp, Quest, and ARUP
- Available RUO assays: FISH, PCR, ELISA
MAPK3
Another member of the MAP kinase family, MAPK3 is being used a clinical trials for a variety of different cancer types.
- Existing test providers: none among Labcorp, Quest, and ARUP
- Available RUO assays: IHC, PCR, ELISA
Hopefully this list provides a useful, if small, starting point for people interested in mining clinical trial records for product development opportunities. New trials and publications involving these biomarkers will provide additional validation for their potential as IVDs or LDTs.





